SCAR – Supplier Corrective Action Report Programs: A Practical Guide Developed by the Supplier Quality Working Group
‘SCAR’ gives pharmaceutical industry professionals a framework for handling improvements that they demand from their suppliers (i.e., vendors) based on regulations and/or the outsourcing entity’s own quality standards. This guide includes a template SCAR form […]
Patient Safety Blog – How to Plan for the Unplannable
Life sciences and healthcare organizations must have comprehensive risk management and crisis management plans that can provide for proactive risk scenario planning and a rapid response to supply chain emergencies. They also need to develop […]
What We Read
Decision making might be tougher now than it has been for most of our careers. Today, we all have unprecedented needs for reliable info sources. “Grassroots” style info sharing is proving to be a critical […]
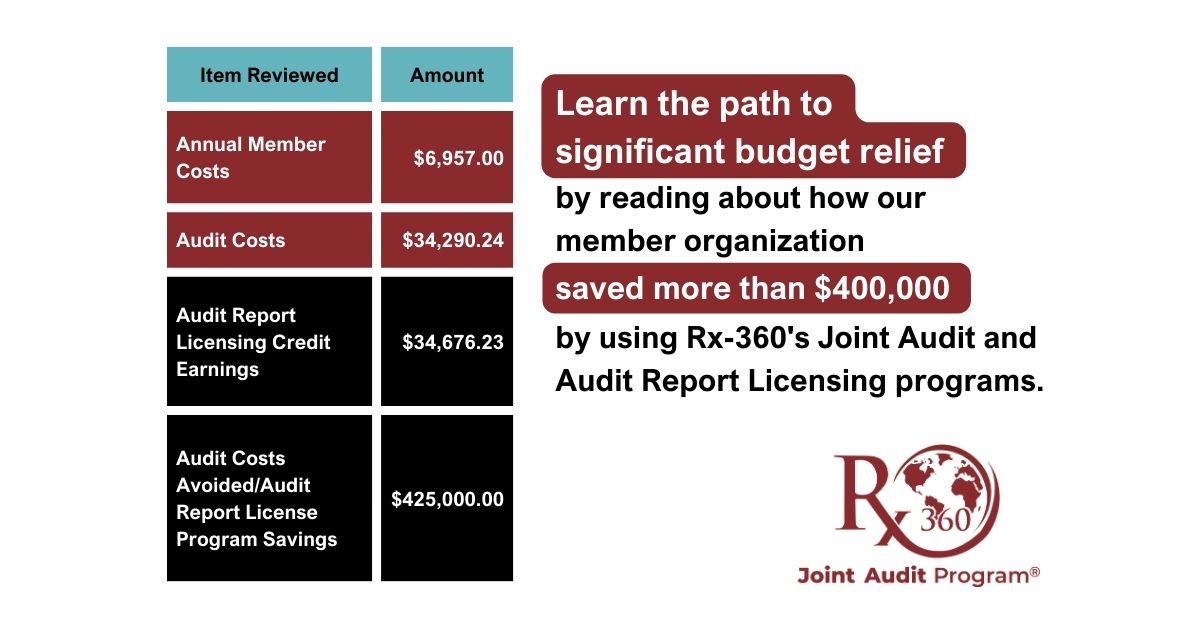
Real Value of Rx-360’s Consortium Membership and Joint Audit Program
Read the 2024 Case Study – showing significant budget relief that came from Rx-360’s programs and consortium membership on a major player in biotech, chemical manufacturing, and life sciences.