Featured Content
Cargo Security Risk Assessment and Planning for Global Operations
This whitepaper provides an overview of industry best practices for developing and implementing an effective conveyance
security program regardless of company size or prior experience with supply chain security issues.
Cargo Security Risk Assessment and Planning for Global Operations
This whitepaper provides an overview of industry best practices for developing and implementing an effective conveyance security program regardless of company size or prior experience with supply chain security issues.
Trending
- Guide to Investigations – A Collaborative Publication by the Rx-360 Latin America Working Group
- Rx-360 Announces Approval as a Pilot Center of Excellence for the APEC Regulatory Harmonization Steering Committee
- Supplier Assessment Questionnaire
- Rx-360 Understanding the Threat of Illicit Medicines: An Overview of Counterfeit, Tampered, Unapproved and Diverted Pharmaceuticals
- Selection and GMP Auditing of Software and Hardware Vendors
- Real Value of Rx-360’s Consortium Membership and Joint Audit Program
Patient Safety Blog – How to Plan for the Unplannable
Get the background on why supply chain security and risk management should be integrated with business strategy. Save our list of “10 Actions to Mitigate Supply Chain Risk”. Read the post.
Patient Safety Blog – What We Read
Check out a few of our favorite go-to online resources that help guide our working group agendas. “Grassroots” style info sharing is proving to be a critical way to stay informed. Rx-360’s member working groups, […]
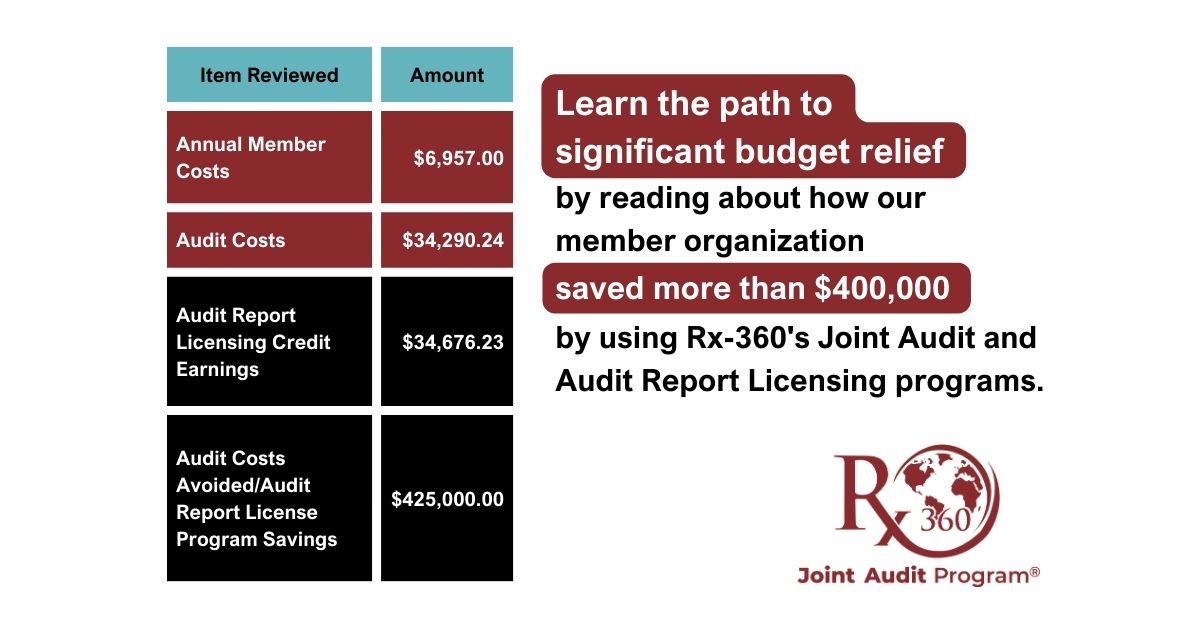
Real Value of Rx-360’s Consortium Membership and Joint Audit Program
Read the 2024 Case Study – showing significant budget relief that came from Rx-360’s programs and consortium membership on a major player in biotech, chemical manufacturing, and life sciences.
Guide to Investigations – A Collaborative Publication by the Rx-360 Latin America Working Group
The purpose of this guide is to provide tools that enable suppliers and service providers of the Healthcare and Pharmaceutical Industry to carry out robust investigations of nonconformities that help determine the impact on the quality of the product involved and consequently, the potential adverse impact for the patient that uses that regulated product. This publication is available in three languages.
Cargo Security Risk Assessment and Planning for Global Operations
This whitepaper provides an overview of industry best practices for developing and implementing an effective conveyance
security program regardless of company size or prior experience with supply chain security issues.
Rx-360 Announces Approval as a Pilot Center of Excellence for the APEC Regulatory Harmonization Steering Committee
Rx-360® is pleased to announce that it has been accepted as a Pilot Center of Excellence (CoE) for the APEC Regulatory Harmonization Steering Committee. The pilot will be focused on the Global Medical Product Supply Chain Integrity Priority Work Area Roadmap.
Supplier Assessment Questionnaire
Background on Supplier Assessment Questionnaire Pharmaceutical manufacturers and industry suppliers recognize the inefficiency and waste of resources when each individual pharmaceutical company creates its own supplier assessment questionnaire. Suppliers routinely exhaust valuable resources continuously completing […]
Rx-360 Announces 2024 Board of Directors
Rx-360® is pleased to announce its 2024 Board of Directors. As a non-profit, the Board of Directors is positioned to continue driving the Rx-360® mission of pharmaceutical supply chain security, material quality, and patient safety. This announcement follows the organization’s successful growth in 2023.
Rx-360 Releases Two New Publications in Support of the Pharmaceutical Industry and Patient Safety
Rx-360 is pleased to announce that the consortium membership led by key members of the Supplier Quality Working Group have released two new papers this week to help drive best practices in pharmaceutical supply chain integrity and patient safety.
Managing Critical Vendors Version 2.0
The manufacture of medical products, drugs and devices is highly regulated all over the world. All aspects of the manufacturing process, from product design to product delivery, require documented procedures. Key points in the process […]