Patient Safety Blog – How to Plan for the Unplannable
Get the background on why supply chain security and risk management should be integrated with business strategy. Save our list of “10 Actions to Mitigate Supply Chain Risk”. Read the post.
Patient Safety Blog – What We Read
Check out a few of our favorite go-to online resources that help guide our working group agendas. “Grassroots” style info sharing is proving to be a critical way to stay informed. Rx-360’s member working groups, […]
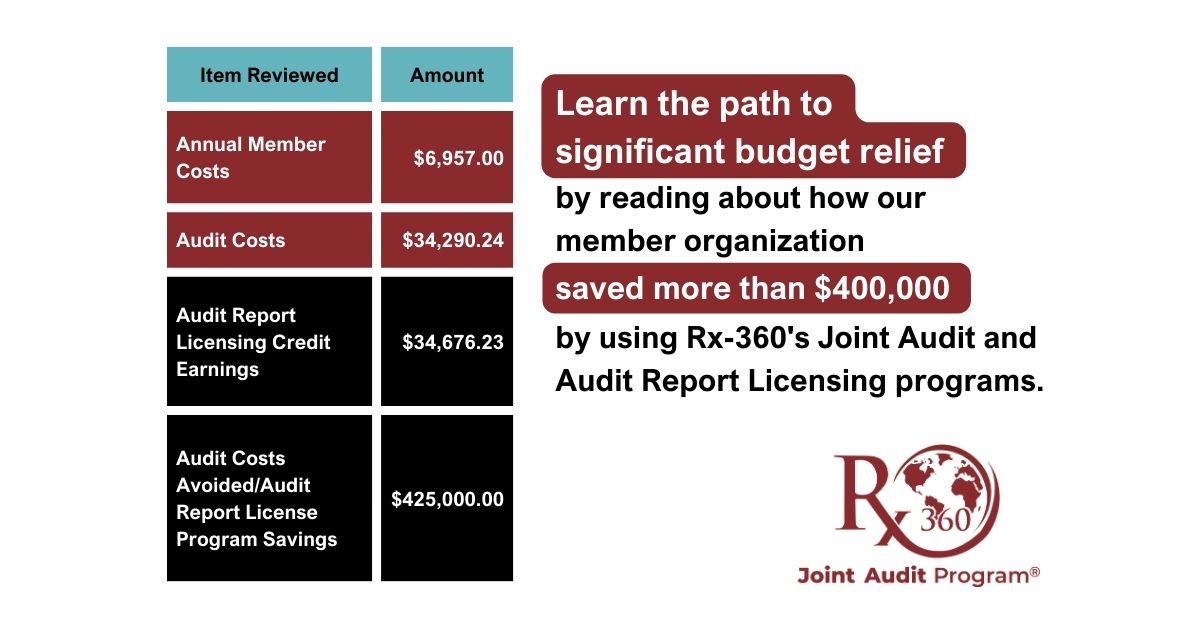
Real Value of Rx-360’s Consortium Membership and Joint Audit Program
Read the 2024 Case Study – showing significant budget relief that came from Rx-360’s programs and consortium membership on a major player in biotech, chemical manufacturing, and life sciences.
Rx-360 Announces Approval as a Pilot Center of Excellence for the APEC Regulatory Harmonization Steering Committee
Rx-360® is pleased to announce that it has been accepted as a Pilot Center of Excellence (CoE) for the APEC Regulatory Harmonization Steering Committee. The pilot will be focused on the Global Medical Product Supply Chain Integrity Priority Work Area Roadmap.
Rx-360 Announces 2024 Board of Directors
Rx-360® is pleased to announce its 2024 Board of Directors. As a non-profit, the Board of Directors is positioned to continue driving the Rx-360® mission of pharmaceutical supply chain security, material quality, and patient safety. This announcement follows the organization’s successful growth in 2023.
Rx-360 Releases Two New Publications in Support of the Pharmaceutical Industry and Patient Safety
Rx-360 is pleased to announce that the consortium membership led by key members of the Supplier Quality Working Group have released two new papers this week to help drive best practices in pharmaceutical supply chain integrity and patient safety.
Achieve Greater Efficiencies in the Supply Chain: An Overview of Key Supplier Quality Questionnaires
Creating efficiencies in the supply chain, especially when it comes to auditing suppliers and manufacturers, is mission critical. With so many moving pieces, it can be difficult to ensure supply chain integrity. That’s where a […]
Improve Supply Chain Security: An Overview of Our Supplier-Led Working Group’s Whitepapers
In the pharmaceutical industry, ensuring the integrity of the supply chain, material quality, and patient safety is paramount. One of the tools that organizations use to achieve these goals is the audit process. Knowing the […]
Rx-360 Releases Supplier Assessment Questionnaire Version 3.0 as a Resource to the Pharmaceutical Industry
Rx-360 is pleased to announce that the consortium membership led by key members of the Supplier Quality Working Group has released Version 3.0 of the Supplier Assessment Questionnaire.
Rx-360 Collaborates with Michigan State University to Offer Course on Pharmaceutical Brand Protection and Supply Chain Integrity
Rx-360 is pleased to announce that the consortium is collaborating with Michigan State University’s Eli Broad College of Business to develop and offer a new educational course on pharmaceutical brand protection and supply chain integrity.