Featured Content
Cargo Security Risk Assessment and Planning for Global Operations
This whitepaper provides an overview of industry best practices for developing and implementing an effective conveyance
security program regardless of company size or prior experience with supply chain security issues.
Cargo Security Risk Assessment and Planning for Global Operations
This whitepaper provides an overview of industry best practices for developing and implementing an effective conveyance security program regardless of company size or prior experience with supply chain security issues.
Trending
- Guide to Investigations – A Collaborative Publication by the Rx-360 Latin America Working Group
- Rx-360 Announces Approval as a Pilot Center of Excellence for the APEC Regulatory Harmonization Steering Committee
- Supplier Assessment Questionnaire
- Rx-360 Understanding the Threat of Illicit Medicines: An Overview of Counterfeit, Tampered, Unapproved and Diverted Pharmaceuticals
- Selection and GMP Auditing of Software and Hardware Vendors
- Real Value of Rx-360’s Consortium Membership and Joint Audit Program
Quality Elements for Suppliers of Products or Services to GMP Regulated Companies
Medical products are items used to diagnose, treat, cure, mitigate or prevent disease in patients. These include pharmaceutical products and medical devices. The manufacture of medical products that are used in the United States, and […]
Working Group Excerpts
Improve Supply Chain Security: An Overview of Our Supplier-Led Working Group’s Whitepapers
In the pharmaceutical industry, ensuring the integrity of the supply chain, material quality, and patient safety is paramount. One of the tools that organizations use to achieve these goals is the audit process. Knowing the […]
Achieve Greater Efficiencies in the Supply Chain: An Overview of Key Supplier Quality Questionnaires
Creating efficiencies in the supply chain, especially when it comes to auditing suppliers and manufacturers, is mission critical. With so many moving pieces, it can be difficult to ensure supply chain integrity. That’s where a […]
Rx-360 Releases Supplier Assessment Questionnaire Version 3.0 as a Resource to the Pharmaceutical Industry
Rx-360 is pleased to announce that the consortium membership led by key members of the Supplier Quality Working Group has released Version 3.0 of the Supplier Assessment Questionnaire.
Rx-360 Collaborates with Michigan State University to Offer Course on Pharmaceutical Brand Protection and Supply Chain Integrity
Rx-360 is pleased to announce that the consortium is collaborating with Michigan State University’s Eli Broad College of Business to develop and offer a new educational course on pharmaceutical brand protection and supply chain integrity.
Rx-360 Expands Trademark Portfolio to Japan
Rx-360 is pleased to announce that the consortium has now expanded their trademark registration for Joint Audit Program® logo to now include Japan.
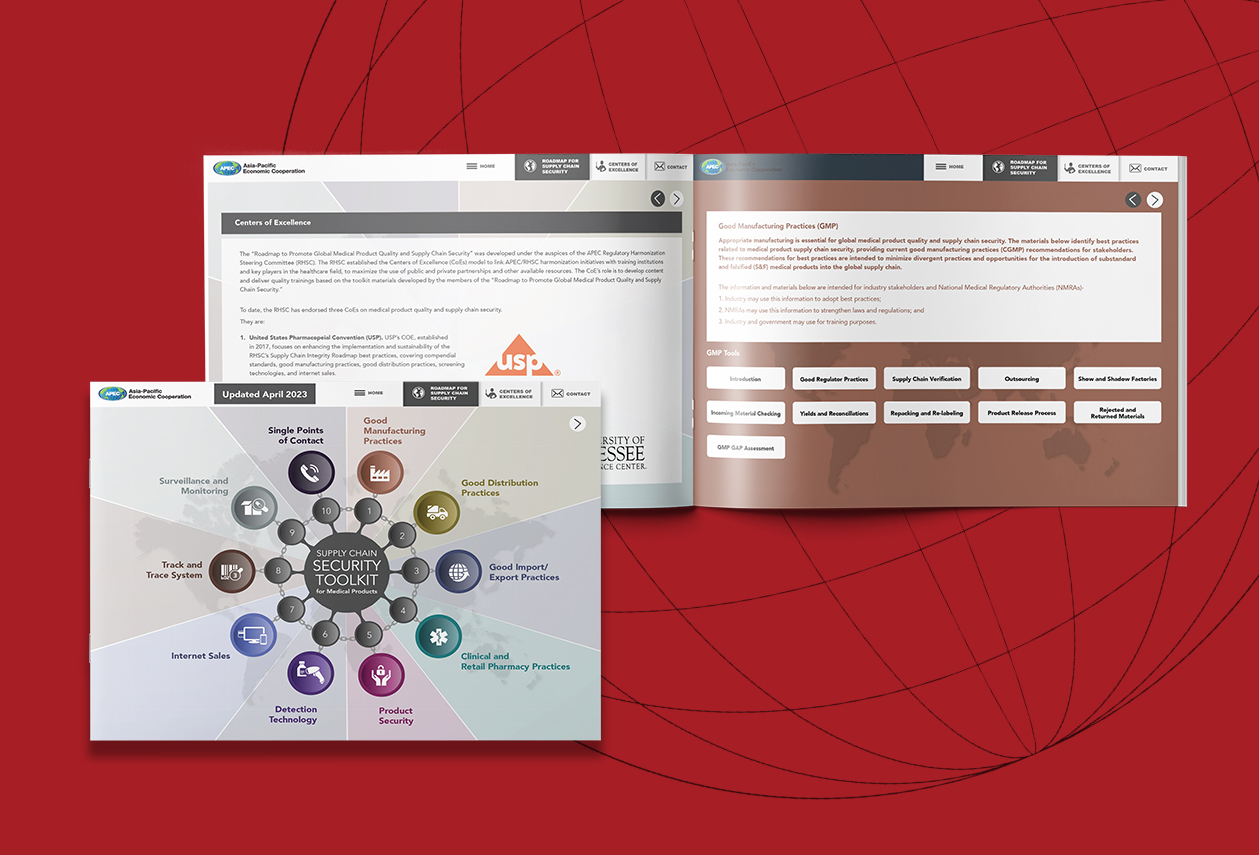
APEC Supply Chain Security Toolkit for Medical Products
Rx-360 has been a promoter of the APEC Supply Chain Toolkit from its founding, which our members played an integral role in. The APEC Supply Chain Security Toolkit is now hosted by the USP. USP […]
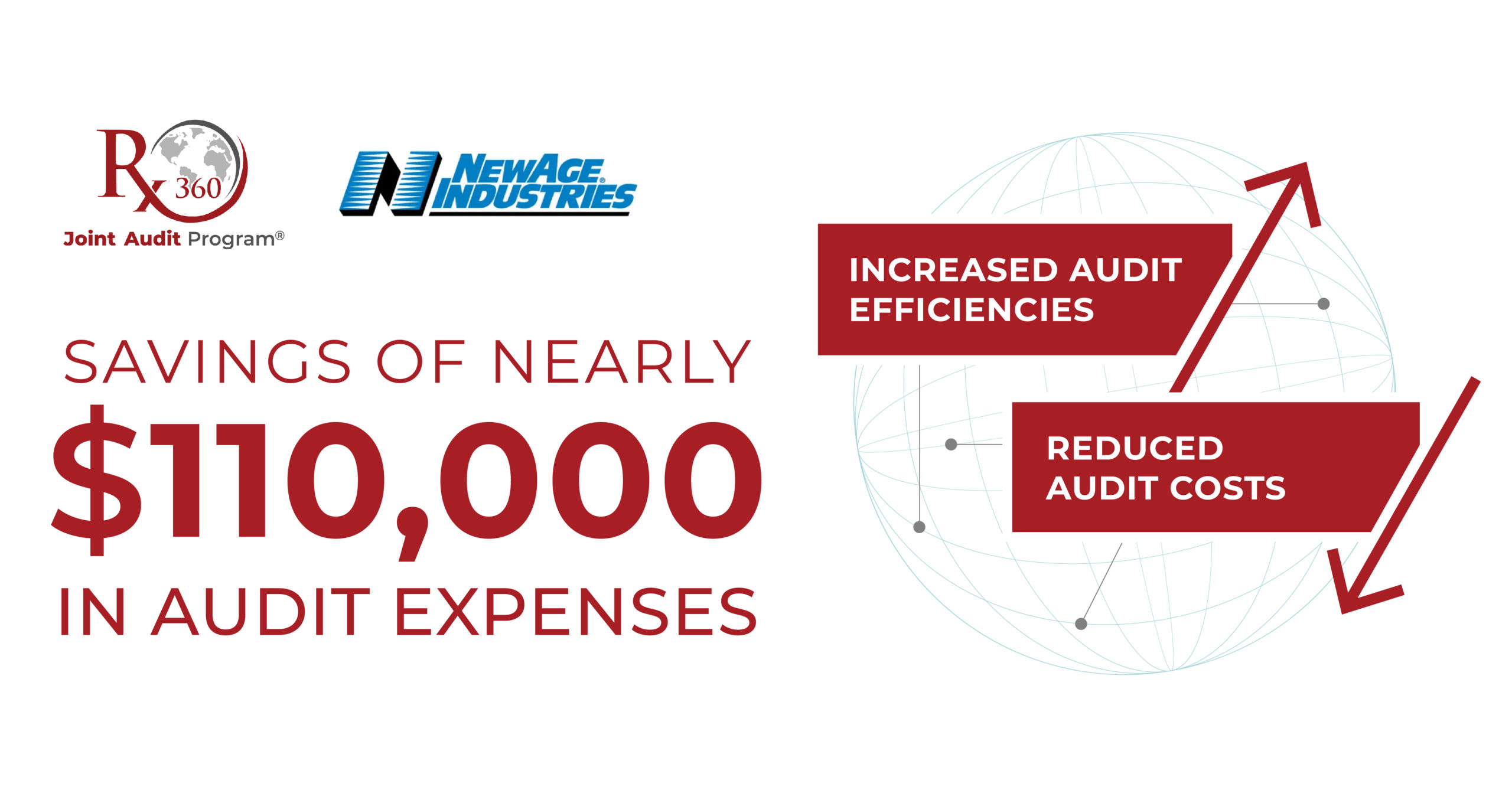
Rx-360 Helps NewAge Industries Reduce Audit Costs and Save Time
NewAge Industries, a member of Rx-360, is a leading independent, employee-owned manufacturer of fluid transfer systems that produces high-quality tubing, hose, fittings, clamps, and accessories and offers expert project guidance and services. NewAge Industries […]
Good Distribution & Warehouse Practices for Warehouse & Transportation Suppliers in LATAM
The warehousing and distribution of regulated medical products is a very important part of the supply chain from manufacturer to the patient. Various separate entities are responsible for the storage and shipping of these products […]