Featured Content
Cargo Security Risk Assessment and Planning for Global Operations
This whitepaper provides an overview of industry best practices for developing and implementing an effective conveyance
security program regardless of company size or prior experience with supply chain security issues.
Cargo Security Risk Assessment and Planning for Global Operations
This whitepaper provides an overview of industry best practices for developing and implementing an effective conveyance security program regardless of company size or prior experience with supply chain security issues.
Trending
- Guide to Investigations – A Collaborative Publication by the Rx-360 Latin America Working Group
- Rx-360 Announces Approval as a Pilot Center of Excellence for the APEC Regulatory Harmonization Steering Committee
- Supplier Assessment Questionnaire
- Rx-360 Understanding the Threat of Illicit Medicines: An Overview of Counterfeit, Tampered, Unapproved and Diverted Pharmaceuticals
- Selection and GMP Auditing of Software and Hardware Vendors
- Real Value of Rx-360’s Consortium Membership and Joint Audit Program
Episode 4: Interview with Leigh Verbois, Director of the Office of Drug Security, Integrity and Response for the FDA
Host Jim Fries speaks to Leigh Verbois, the Director of the Office of Drug Security, Integrity, and Response for the FDA, in the fourth episode of The Patient Safety Podcast. The episode covers Verbois’ time […]
Human Blood Origin Materials for Cell & Gene Therapy Manufacturing Supplier Questionnaire
Materials derived from human blood such as human serum albumin and transferrin are frequently used either as components of cell culture medium or in other processes. Suppliers of such reagents have incorporated multiple mitigation steps such as sourcing pharmaceutical grade or licensed drug product, sourcing from safe geographical regions, testing for contaminants and inactivation. In addition, this information needs to be available for review during inspections and in regulatory filings. This questionnaire is a comprehensive list of those requirements and will be a useful document for reference.
Meet The Board: Bill Campagna, Eli Lilly and Company
Bill Campagna is the Senior Director of Quality for Procurement and Operations for Eli Lilly and Company; and he’s passionate about serving on Rx-360’s Board of Directors. “I’m proud to be part of Rx-360’s Board […]
Rx-360 Patient Safety Podcast: Interview with USP CEO Ron Piervincenzi, Ph.D.
Rx-360 released the third episode of The Patient Safety Podcast, featuring Ron Piervincenzi, Ph.D., CEO of the United States Pharmacopeia (USP).
Episode 3: Interview with Ron Piervincenzi, CEO of The United States Pharmacopeia
Host Jim Fries speaks to Ron Piervincenzi, Ph.D., CEO of the United States Pharmacopeia (USP), in the third episode of The Patient Safety Podcast. The episode covers topics such as Piervincenzi’s time at USP, the […]
Episode 2: Interview with Dr. Janet Woodcock, Principal Deputy Commissioner of the FDA
For the second episode of the Patient Safety Podcast, host Jim Fries, CEO of Rx-360, speaks to Dr. Janet Woodcock, Principal Deputy Commissioner of the Food and Drug Administration. Throughout the course of their conversation, […]
Rx-360 Launches New Podcast: The Patient Safety Podcast
Rx-360 has released the first episode of The Patient Safety Podcast, a new series that explores patient safety in the pharmaceutical ecosystem.
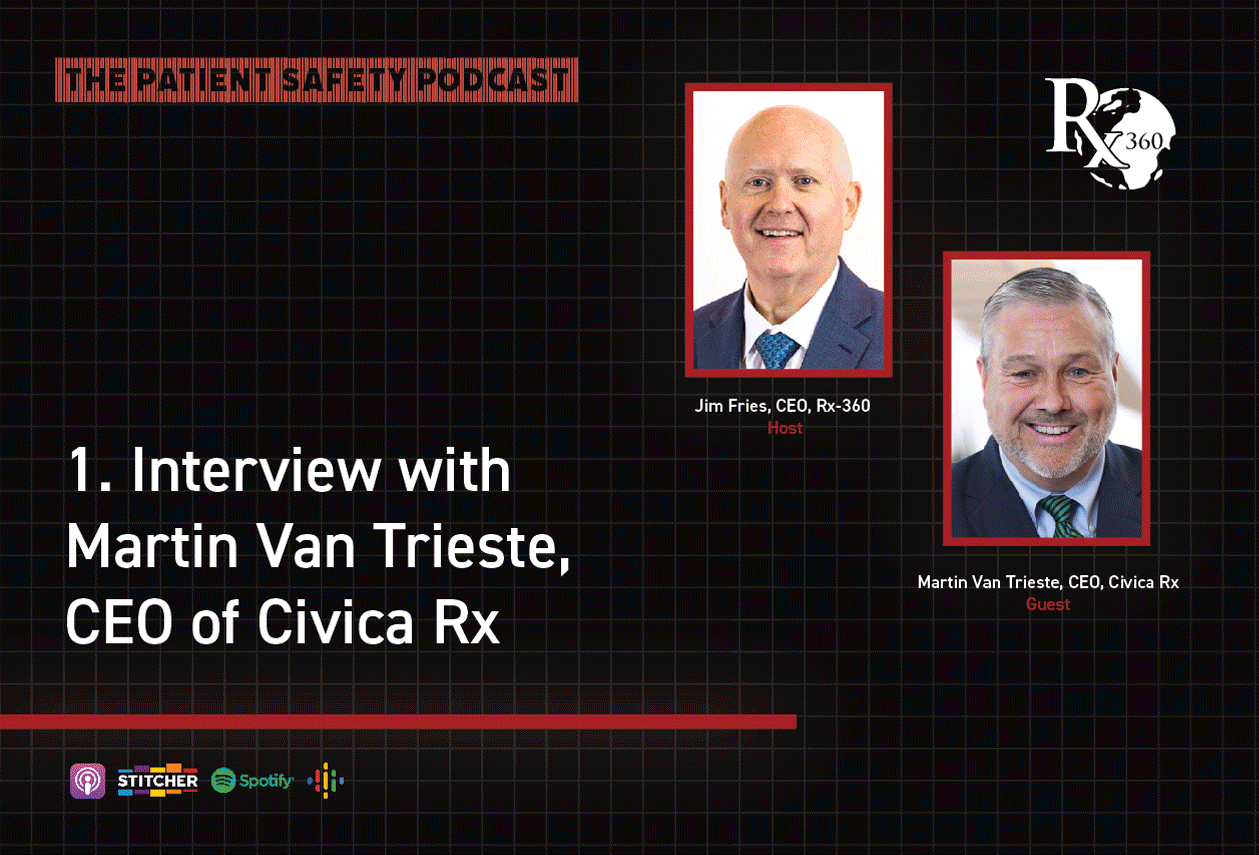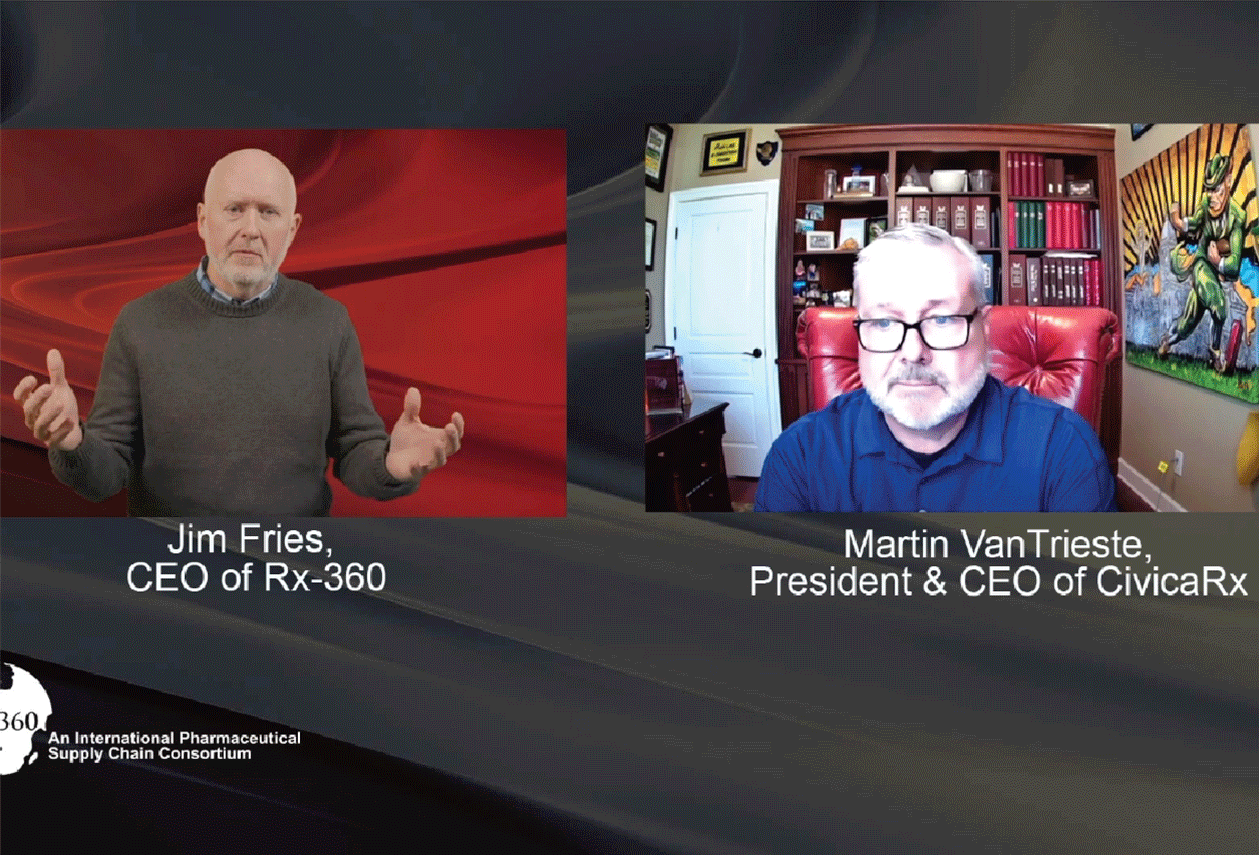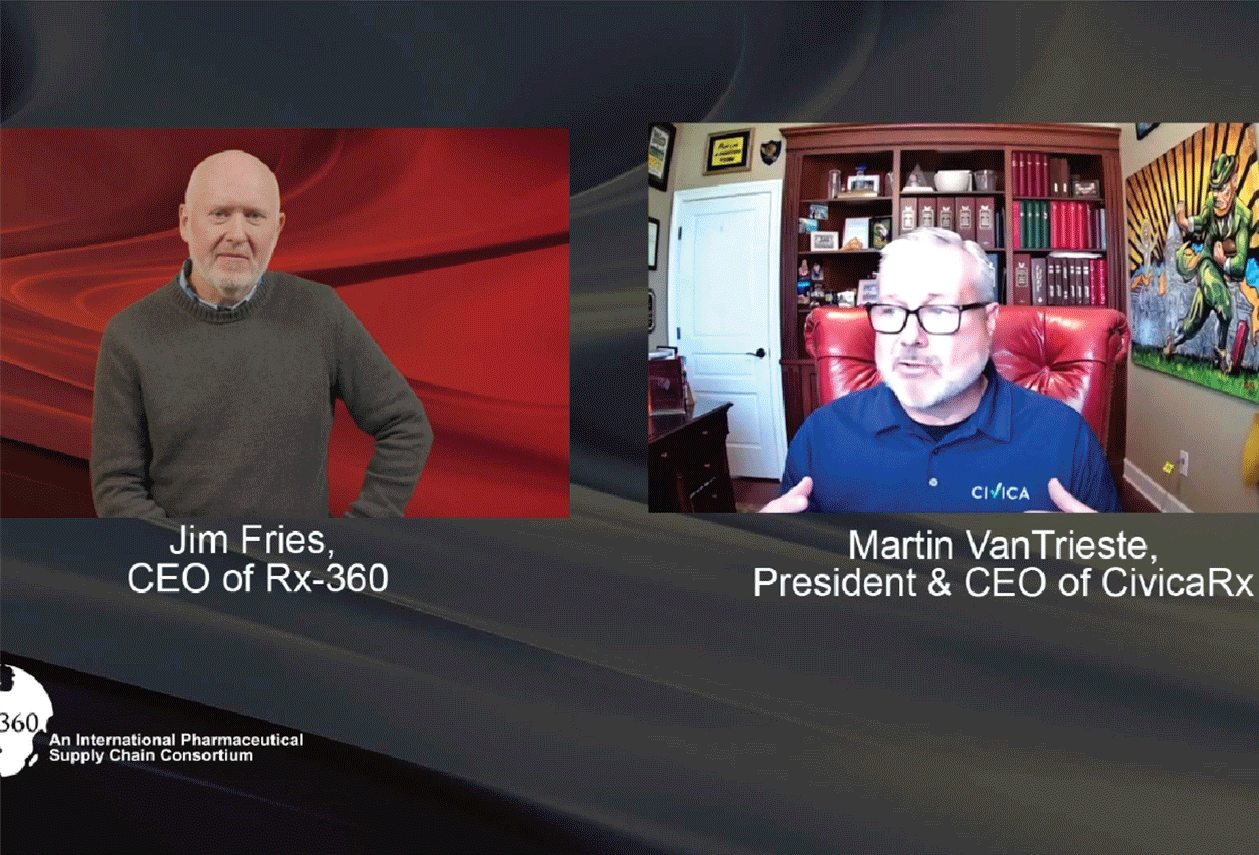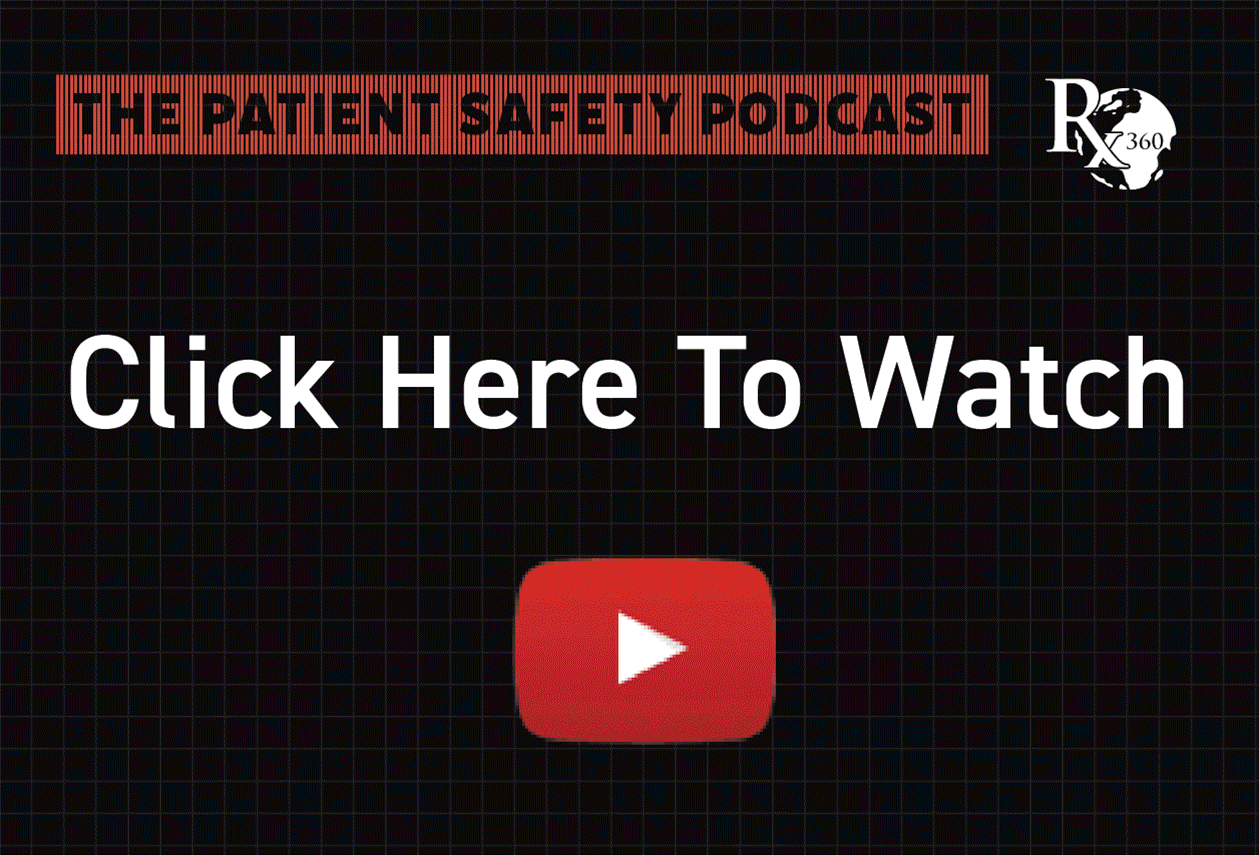
Episode 1: Interview with Martin Van Trieste, CEO of Civica Rx
Host Jim Fries, CEO of Rx-360, speaks to Martin Van Trieste, president and CEO of Civica Rx in the first episode of the Patient Safety Podcast. Covering topics such as the origin story of Rx-360 […]
Rx-360 Announces 2022 Board of Directors
Rx-360 is pleased to announce their 2022 Board of Directors.
Rx-360 Launches New Interactive Website
Rx-360 has announced the launch of its new website at Rx-360.org.