Patient Safety Blog – How to Plan for the Unplannable
Get the background on why supply chain security and risk management should be integrated with business strategy. Save our list of “10 Actions to Mitigate Supply Chain Risk”. Read the post.
Patient Safety Blog – What We Read
Check out a few of our favorite go-to online resources that help guide our working group agendas. “Grassroots” style info sharing is proving to be a critical way to stay informed. Rx-360’s member working groups, […]
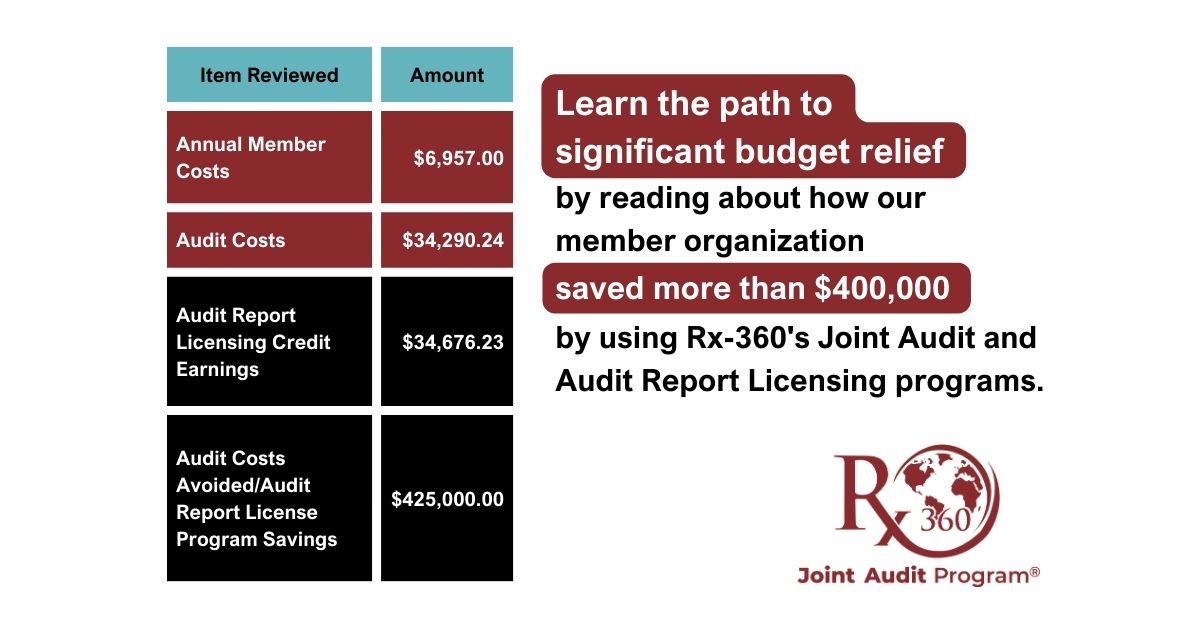
Real Value of Rx-360’s Consortium Membership and Joint Audit Program
Read the 2024 Case Study – showing significant budget relief that came from Rx-360’s programs and consortium membership on a major player in biotech, chemical manufacturing, and life sciences.
Guide to Investigations – A Collaborative Publication by the Rx-360 Latin America Working Group
The purpose of this guide is to provide tools that enable suppliers and service providers of the Healthcare and Pharmaceutical Industry to carry out robust investigations of nonconformities that help determine the impact on the quality of the product involved and consequently, the potential adverse impact for the patient that uses that regulated product. This publication is available in three languages.
Cargo Security Risk Assessment and Planning for Global Operations
This whitepaper provides an overview of industry best practices for developing and implementing an effective conveyance
security program regardless of company size or prior experience with supply chain security issues.
Supplier Assessment Questionnaire
Background on Supplier Assessment Questionnaire Pharmaceutical manufacturers and industry suppliers recognize the inefficiency and waste of resources when each individual pharmaceutical company creates its own supplier assessment questionnaire. Suppliers routinely exhaust valuable resources continuously completing […]
Managing Critical Vendors Version 2.0
The manufacture of medical products, drugs and devices is highly regulated all over the world. All aspects of the manufacturing process, from product design to product delivery, require documented procedures. Key points in the process […]
Quality Elements for Suppliers of Products or Services to GMP Regulated Companies
Medical products are items used to diagnose, treat, cure, mitigate or prevent disease in patients. These include pharmaceutical products and medical devices. The manufacture of medical products that are used in the United States, and […]
Good Distribution & Warehouse Practices for Warehouse & Transportation Suppliers in LATAM
The warehousing and distribution of regulated medical products is a very important part of the supply chain from manufacturer to the patient. Various separate entities are responsible for the storage and shipping of these products […]